ADAPT™
(Assay for Discovery of Antagonist Peptides based on Transcription)
ADAPT reinvents peptide drug discovery. Unlike other discovery approaches, ADAPT discovers in a cellular environment and tests bespoke peptide libraries for drug candidates that may disrupt full length, properly folded, protein-protein interactions (PPIs).
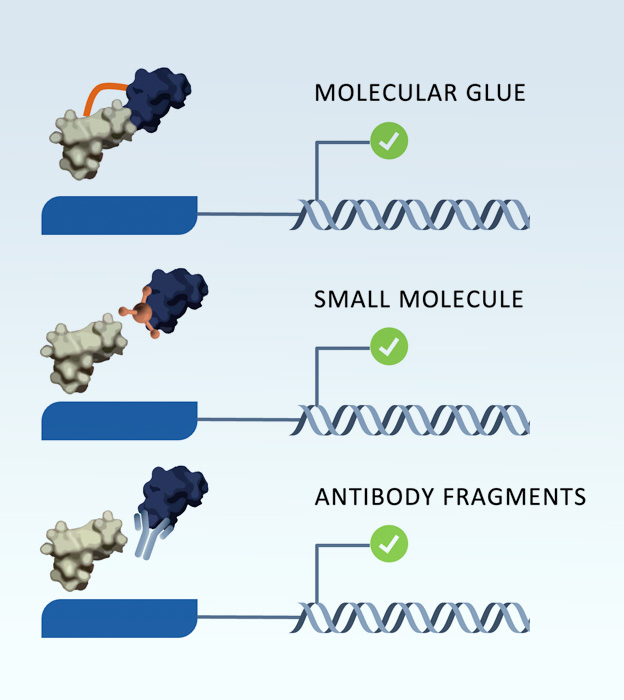
ADAPT is a smart platform that was developed by Sapience scientists with many inherent functionalities, providing for a quantifiable and tunable system. Beyond peptide discovery, ADAPT is amenable to screening other types of molecules, including small molecules, antibody fragments and molecular glues.
ADAPT’s Key Attributes
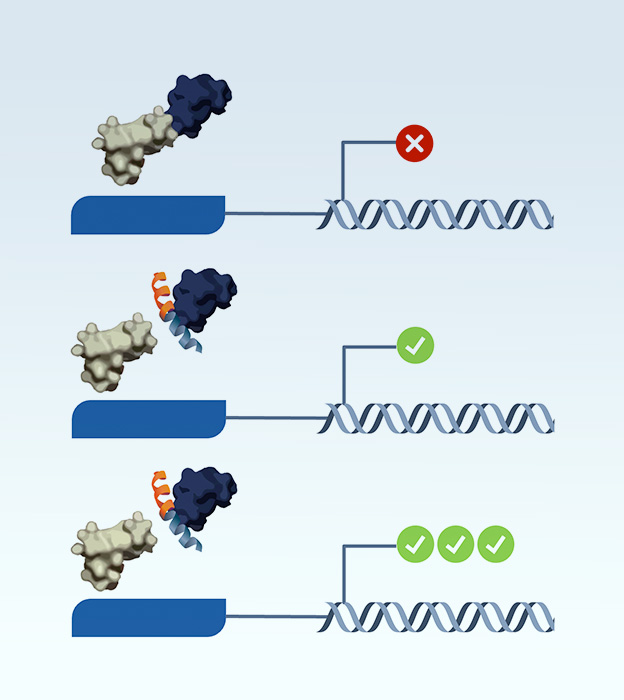
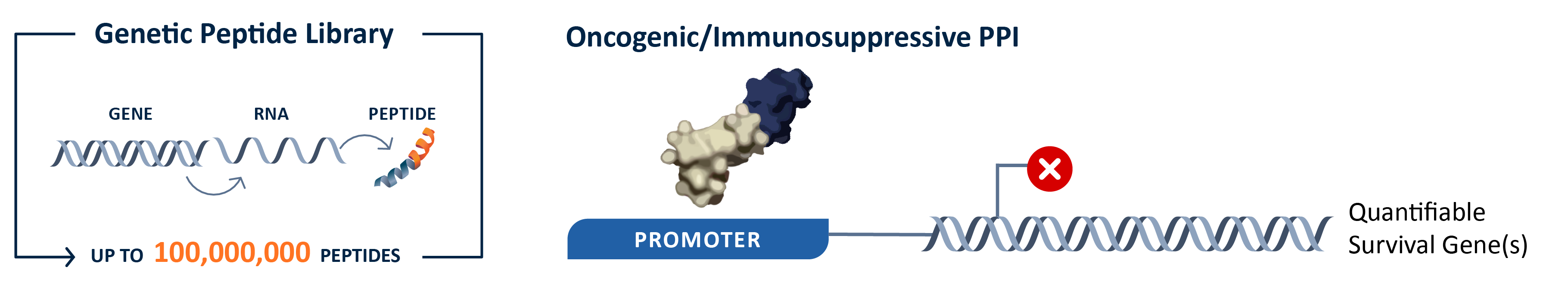
By quantifying the expression levels of the reporter gene, our platform determines the interaction strengths between protein complex disruptor and the target. Gene expression quantification across conditions in the system serves as a powerful lens into transient interactions, strength determination, and modifier identification.
Our platform is adaptable to the discovery of diverse classes of protein interaction modulators. The system has the potential to identify compounds capable of disrupting key protein-protein interfaces as well as discovering molecular glues.
We screen peptides against targets in their properly folded states. By optimizing codon usage, balancing solubility and expressibility, and tightly regulating co-translational folding in our proprietary strains, we reliably discover modulators that preserve specificity and molecular properties across various phases of development. The system ensures eliminations of nonspecific and toxic compounds prior to their progression into downstream development processes.
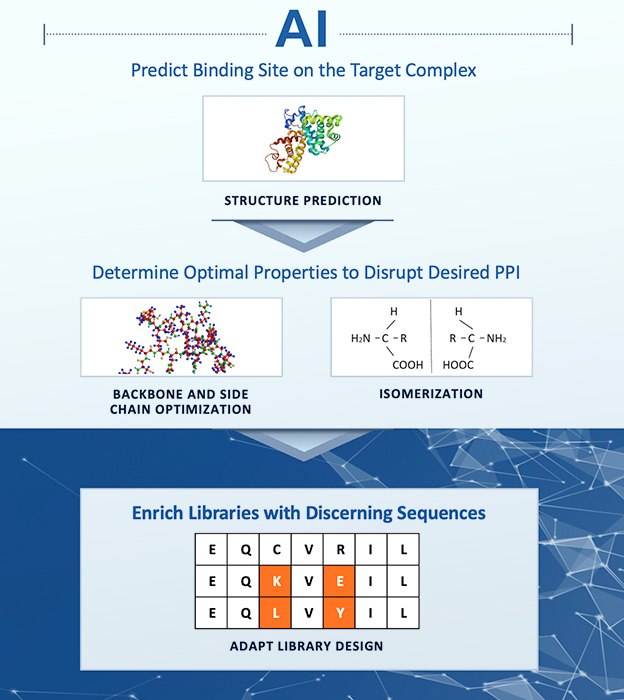
Leveraging AI to predict binding sites on target protein complexes, our platform intelligently biases molecular library designs towards improved outcomes, expediting iterative optimizations of compound backbones, sidechains, and stereoisomers for enhanced potency and selectivity.
PADS™
(Peptide Antagonist Discovery System)
PADS starts with a library-based protein fragment complementation screen. This step of PADS is performed in an intracellular environment, ensuring that our peptide hits are more likely to be resistant to degradation by proteases, be soluble, nontoxic, and target-specific in the presence of other cytoplasmic proteins. Initial hits are distilled to leads through rational design by our team of scientists.
Design the Screen
- Identify PPI of interest
- Design semi-rational multi-million-member peptide library to screen against target
Run the Screen
- In vitro expression system used to screen library
- Successful campaign identifies a single ‘hit’ peptide for further development
Develop Hits into Therapeutic Leads
- Sapience peptide chemists use the hit as the scaffold to design a small, rational peptide library optimizing for manufacturability, solubility, stability, immunogenicity, and activity